Abstract
Introduction: Patients with myeloproliferative neoplasms (MPN) are at increased risk for cardiovascular morbidity and mortality with the highest risk among polycythemia vera (PV) patients. MPN guidelines recommend aggressive management of cardiovascular risk factors which may include statin administration. Statins can induce apoptosis and inhibit JAK2-V617F-dependent cell growth in vitro, as well as inhibit erythropoietin-independent erythroid colony formation of primary cells from patients with MPN. JAK2-V617F mutation is a common somatic event occurring in over 95% of PV patients. There is no published data regarding the impact of statins on the outcomes of PV patients.
Methods: Using the Surveillance, Epidemiology, and End Results-Medicare linked database, we assembled a population-based cohort of older adults who were diagnosed with PV during 2007-2013 and fulfilled the following eligibility criteria: 1) aged 66-99 years at diagnosis; 2) had continuous Medicare Parts A and B coverage and not enrolled in health maintenance organizations from 12 months before diagnosis to death or the end of study (12/31/2014), whichever was earlier (i.e., the end of follow up); and 3) continuously enrolled in Medicare Part D from 6 months before diagnosis to end of follow up. Patients were identified as statin users if they had any prescription for statins in Part D claims from their PV diagnosis to the end of follow up. For both statins and hydroxyurea (HU, a common treatment of PV), percentage of days covered (PDC) was defined as the percentage of days from diagnosis to the end of follow-up covered by respective prescriptions. We further classifed statins as lipophilic or hydrophilic. Therapeutic phlebotomy intensity was defined based on the number of phlebotomies per year (>0 but < 3: low intensity, ≥3: high intensity).
Log-rank tests were used to compare Kaplan-Meier (K-M) curves between statin users and non-users with regard to overall survival and risk of thrombosis after PV diagnosis. Multivariate Cox proportional hazards models were used to assess the impact of statins use on overall survival. Multivariate competing risk models with death as a competing risk were utilized to evaluate the relationship between statin PDC and thrombosis risk. All multivariate models adjusted for HU PDC, phlebotomy intensity, age, sex, race, state buy-in, influenza vaccination 12 months prior to PV diagnosis (a marker for healthcare access), disability status, modified Elixhauser score for comorbidities, and previous thrombosis. All statistical tests were two-sided and conducted with SAS (Version 9.4).
Results: Of the 721 PV patients included in this study (median age = 77 years, interquartile range: 71-83 years), a majority were female (56.7%) and white (91.4%) (Table 1). About 55% of patients used statins, and 72.9% of the 395 users (n=288) started before PV diagnosis. The median statin PDC was 67% among statin users, and most patients (n=361, 91.4%) used only one type of statins (lipophilic: n=295; hydrophilic: n=65).
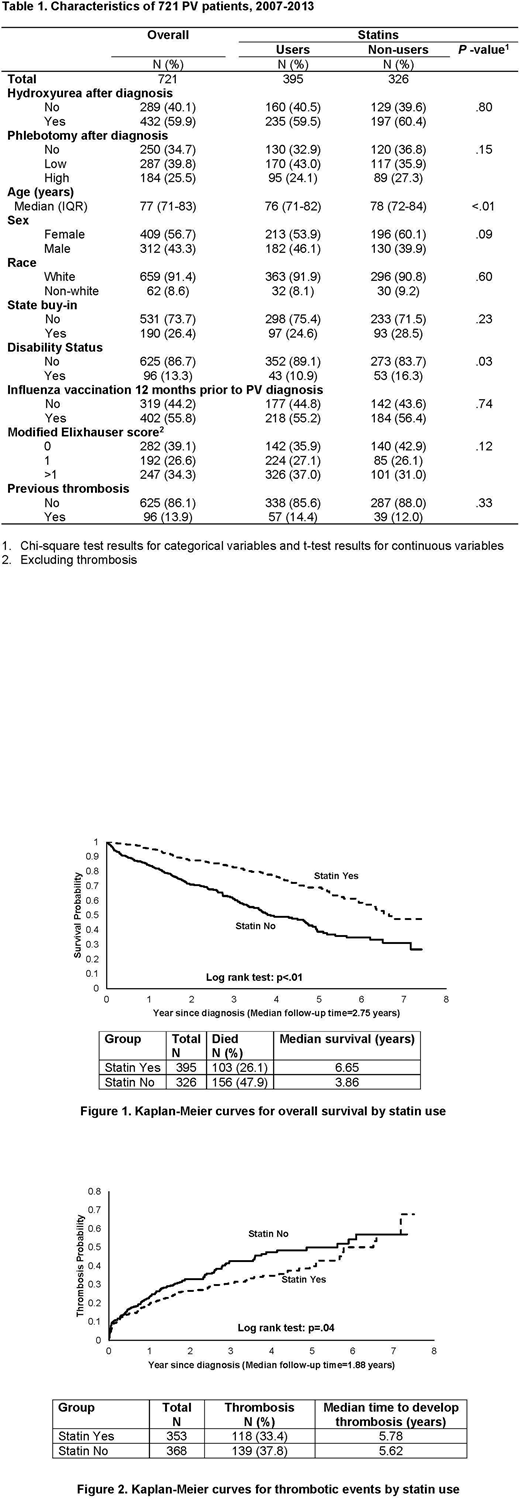
With a median follow-up of 2.75 years, 26.1% (n=103) of statin users and 47.9% (n=156) of statin non-users died; users (median: 6.65 years) had a significantly better overall survival than non-users (median: 3.86 years Log rank test, p<.01) (Figure 1). Adjusting for covariates, every 10% of statin PDC after PV diagnosis was associated with an 18% lower risk of all cause mortality (95% confidence interval [CI]: 0.78-0.86; p<.01). The magnitude of association was very similar for lipophilic and hydrophilic statins.
Thrombosis after PV diagnosis was recorded in 257 (35.6%) patients, 177 of whom had arterial thrombosis. One hundred and eighteen (33.4%) statin users and 139 (37.8%) non-users had thrombosis after PV diagnosis. The risk of thrombosis was significantly lower in statin users than in non-users based on Log-rank test (p=.04) (Figure 2) and multivariable competing risk model (every 10% of statin PDC was associated with a 5% lower risk of thrombosis (95% CI: 0.92-0.99; p<.01). Subgroup analyses for lipophilic and hydrophilic statins generated almost identifical results.
Sensitivity analyses that only included patients who started using statins after PV diagnosis revealed similar findings pertaining to both survival and thrombosis.
Conclusions: In this large population-based cohort study of older adults with PV, use of statins was associated with improved survival and decreased risk of thrombosis.
Podoltsev:Alexion: Consultancy, Honoraria; Astellas Pharma: Research Funding; Sunesis Pharmaceuticals: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding; Celgene: Research Funding; CTI biopharma: Research Funding; Boehringer Ingelheim: Research Funding; Celator: Research Funding. Zeidan:Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Takeda: Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Agios: Consultancy, Honoraria. Davidoff:Celgene: Research Funding. Huntington:Janssen: Consultancy; Bayer: Consultancy; Celgene: Consultancy. Gore:Celgene: Consultancy, Research Funding. Ma:Incyte: Consultancy; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal